The use of autologous bone taken from another part of the body of the patient remains the gold standard treatment for the repair of large bone defects.
That is despite the impact on the patient, the damage done to the donor site and the associated costs and risks of two surgical procedures, one to harvest bone and the second to repair the defect.
Our group is focused on finding alternative tissue engineering based approaches to bone defect repair, focusing on cells, biomaterials and growth factors. We collaborate with many research groups both nationally and internationally on this topic, including a strong collaboration with the Technical University of Delft and have several projects running on the topic.
We are mainly focused on two aspects:
1. increasing our understanding of how to modulate endochondral ossification
2. testing combinations of cells, novel molecules and smart biomaterials for their ability to induce and support bone formation
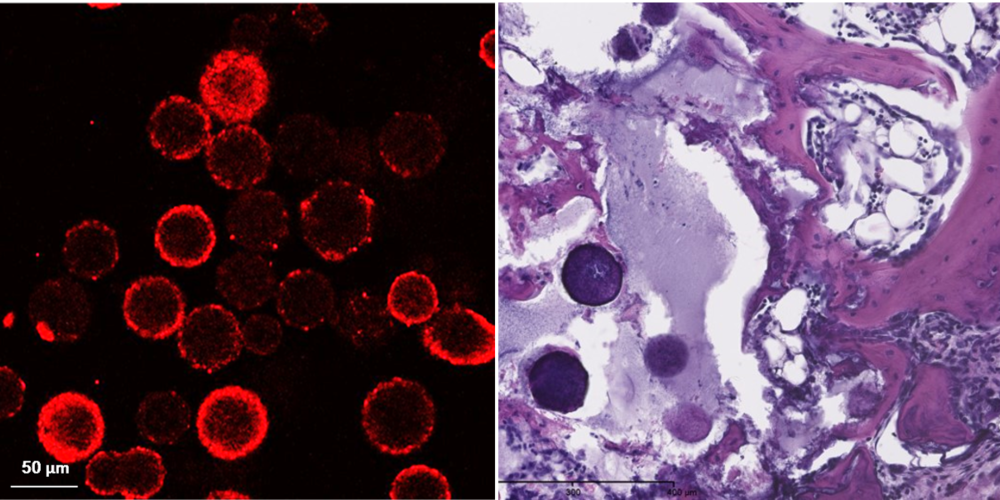
Image: enhancement of in vivo bone formation by delivery of microspheres loaded with growth factor (BMP2) [Fahmy-Garcia et al., Adv Healthc Mater 2018]
People
Anita Jose, Tess Uphof, Shorouk Fahmy Garcia (Alumnus), Enrique Andres Sastre (Alumnus), Yannick Nossin (Alumnus), Callie Knuth (Alumnus)
Traumatic injuries to the osteochondral tissues of diarthrodial joints like the knee result in pain, functional impairment, and increased risk of developing post-traumatic osteoarthritis (PTOA) and its…
Read MoreThe current gold-standard treatment for large bone defects involves autologous transplantation, despite its associated risks, such as donor site morbidity, increased risk of infection, and limited tissue…
Read MoreNANO-SCORES wants to revolutionize the treatment of knee osteochondral lesions, a particular kind of defect that affects cartilage and the underlying bone tissue. This objective will be achieved by developing…
Read MoreCartilage and bone are inextricably linked during development, pathology and repair. During skeletal development most bones of the body are formed via a cartilage intermediate through the process of endochondral…
Read MoreFahmy-Garcia, S., Farrell, E., Witte-Bouma, J., Robbesom-van den Berge, I., Suarez, M., Mumcuoglu, D., Walles, H., Kluijtmans, S., van der Eerden, B. C. J., van Osch, G., van Leeuwen, J., and van Driel, M. (2019)
Frontiers in bioengineering and biotechnology 7, 38
Knuth, C. A., Andres Sastre, E., Fahy, N. B., Witte-Bouma, J., Ridwan, Y., Strabbing, E. M., Koudstaal, M. J., van de Peppel, J., Wolvius, E. B., Narcisi, R., and Farrell, E. (2019)
Eur Cell Mater 38, 106-122
Narcisi, R., and Farrell, E. (2019)
Frontiers in bioengineering and biotechnology 7, 8
Andres Sastre, E., Zaucke, F., Witte-Bouma, J., van Osch, G., and Farrell, E. (2020)
Cartilage, 1947603520961170
Nossin, Y., Farrell, E., Koevoet, W., Somoza, R. A., Caplan, A. I., Brachvogel, B., and van Osch, G. (2020)
Frontiers in bioengineering and biotechnology 8, 327