Cartilage and bone are inextricably linked during development, pathology and repair. During skeletal development most bones of the body are formed via a cartilage intermediate through the process of endochondral ossification. In the context of bone healing, endochondral ossification is also critically important. Normally, bone fractures are healed mainly via this process: the defect is bridged by a cartilage template that later mineralises and is replaced by bone.
The pathobiology of impaired bone healing and osteoarthritis is influenced by cell fate decisions. These ultimately dictate whether cartilage tissue in the healing bone defect site or in the articular joint becomes vascularised and mineralised or not. This control is mediated by intrinsic and extrinsic factors, i.e. cell phenotype, local paracrine factors, the mechanical environment and extracellular matrix molecules, and these determine whether the endochondral ossification pathway is activated or suppressed.
In developmental biology these same factors have been identified as being important; paracrine factors (such as vascular endothelial growth factor (VEGF), fibroblast growth factors (FGF) and Wnt), specific matrix molecules (such as matrilins and cartilage oligomeric matrix protein) and mechanical loading have been shown to be critical in the processes of cartilage formation and endochondral ossification. Furthermore, tissue engineering approaches have recently started to take into account the principles of developmental biology.
When aiming to generate either bone or cartilage, tissue engineers also face this chondrocyte (cartilage cell) fate decision from two perspectives. Cartilage tissue engineers face the challenge of creating stable permanent articular cartilage using mesenchymal stem cells (MSCs) in which the endochondral ossification process is prevented. Conversely, bone tissue engineers have adapted to using the endochondral ossification process for bone re-generation by stimulating MSCs to form a cartilage intermediate, which, upon implantation, becomes vascularised and mineralised and eventually forms bone, albeit of limited quantity.
When bone healing is impaired, the defects are characterised by the formation of cartilage tissue that fails to undergo full endochondral ossification. At the joint surface, in the absence of disease, cartilage is a stable tissue that provides the mechanical environment required for healthy joint motion. However, in osteoarthritis, the articular cartilage fails to remain stable. Undesirable endochondral ossification occurs whereby the cartilage becomes vascularised, mineralised and is eventually replaced by bone. This occurs in the articular cartilage layer as well as on the joint margins where osteophytes, bony outgrowths strongly associated with joint pain, are formed.
CarBon will train 14 high potential scientists to combine knowledge of cartilage & bone developmental biology, pathobiology and tissue engineering with skills in cell culture, animal models, proteomics, biomaterial development, bioreactors and computational modelling.
Exchange of knowledge and multidisciplinary collaboration between these fields of research will raise the next generation of researchers with the skills, multidisciplinary knowledge and on-the-job training experience necessary to tackle all aspects of bone and cartilage disease and repair.
CarBon will identify: the specific factors secreted by chondrogenic cells, the effect of components of the cartilage extra-cellular matrix and the interactions with the mechanical environment that determine whether cartilage either undergoes endochondral ossification or resists these processes and maintains stable cartilage.The research objective of the Cabon project are; i) To increase understanding of the role and interplay of cell secreted factors, extracellular matrix components and mechanical loading in cartilage and bone formation and repair by combining knowledge and skills from developmental biology, pathobiology and biomedical engineering ii) To use the combined knowledge and skills of matrix biology and tissue engineering to develop novel, biologically inspired biomaterials for the formation of stable cartilage and vascularised bone and iii) To use knowledge of cell biology, proteomics, mechano- and pathobiology integrated by computational modelling to identify and to pursue drug targetable components for bone healing and osteoarthritis.
CarBon Website
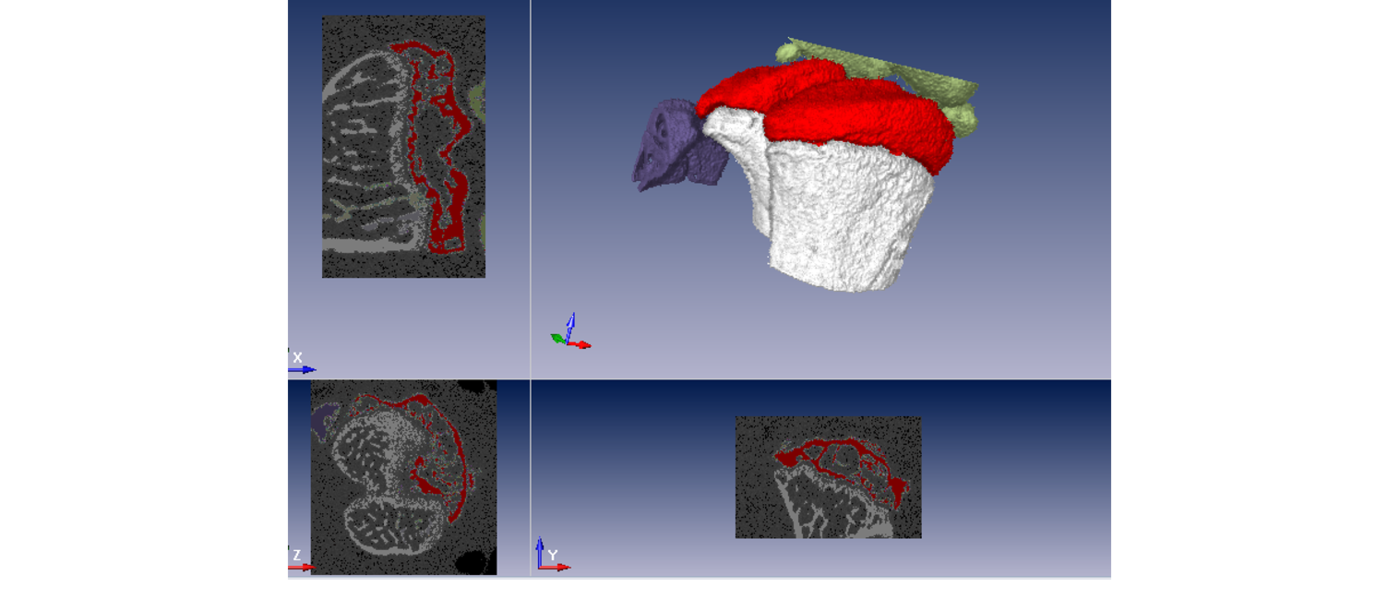
Image: micro-computed tomography (uCT) analysis of mouse knee joint (author: Mauricio Ferrao Blanco)