Endochondral ossification is the process of developmental bone formation that occurs via a cartilage intermediate. It is an extremely complex event that involves multiple cell types, including bone cells, vessel cells and cells of the bone marrow niche. Currently it is not possible to recapitulate endochondral ossification under controlled conditions in the laboratory. This process is not only crucial for bone development and repair after injury, but it also plays a role in several debilitating diseases including osteoarthritis. Furthermore, the long bones (formed via endochondral ossification) are a frequent site of cancer metastasis. The lack of in vitro models to emulate endochondral ossification, prevents us from understanding various disease mechanisms and how to induce bone formation and repair. The ability to model aspects of endochondral ossification in vitro would provide researchers with new tools to investigate the cellular and molecular basis of bone formation under physiological or pathological conditions.
Our group wants to develop advanced in vitro models that can reflect the complexity of vascularised bone. Taking inspiration from the natural process of endochondral ossification, we aim to generate experimental systems that accurately capture aspects of the bone formation process. Ultimately this can provide invaluable tools for fundamental research and drug screening for musculoskeletal diseases and bone-related conditions. Finally, a reliable in vitro model of bone formation will reduce or eliminate the currently unavoidable need for animals to generate bone for research purposes.
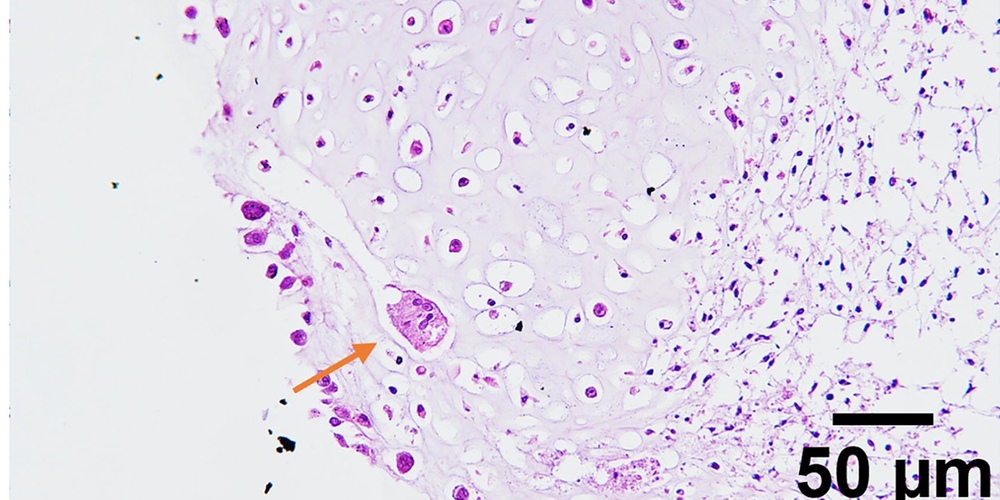
Image: Invasion of MSC pellets by osteoclasts [A. Garmendia Urdalleta et al., Tissue Engineering 2025]
People
Amaia Garmendia Urdalleta, Imke Jansen, Encheng Ji (Alumnus)
BACKGROUND Breast cancer hits 1 woman in 8, and its most common metastatic site is the bone (70%). A major hurdle to overcome breast cancer mortality is the lack of understanding of the dynamics leading…
Read MoreDeciphering bone formation to model diseases: towards a new generation of in vitro bone modelsIn this project, we propose a radically new strategy to develop an urgently needed in vitro model of bone formation.…
Read MoreBone metastasis “in a dish”: engineering vascularised bone in vitro to model metastatic breast cancer; BoneMeND With this new partnership, Erasmus MC, University of Twente and the company React4life…
Read MoreAndres Sastre, E., Maly, K., Zhu, M., Witte-Bouma, J., Trompet, D., Bohm, A. M., Brachvogel, B., van Nieuwenhoven, C. A., Maes, C., van Osch, G., Zaucke, F., and Farrell, E. (2021)
Bone 150, 115999